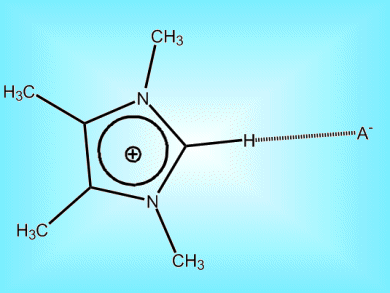
Understanding the balance of bonds that dictate the behavior of room temperature ionic liquids holds the promise of exploiting them further.
Room temperature ionic liquids (ILs) have low toxicity, low volatility and no flammability. As such, they are potential alternatives to volatile organic solvents. Their behavior depends on the subtle balance between Coulomb forces, hydrogen bonds, and dispersion forces among the constituent ions. Spectroscopic research by Ralf Ludwig and co-workers, Universität Rostock, Germany, on newly synthesized imidazolium-based ionic liquids reveal that, while Coulombic forces dominate, hydrogen bonds have significant influence on IL structure and properties. This means that hydrogen bonds could be tuned to expand the working range of IL viscosities and melting point ranges.
- The influence of hydrogen bonding on the physical properties of ionic liquids
K. Fumino, T. Peppel, M. Geppert-Rybczynska, D. H. Zaitsau, J. K. Lehmann, S. P. Verevkin, M. Köckerling, R. Ludwig,
Phys. Chem. Chem. Phys. 2011.
DOI: 10.1039/C1CP20732F