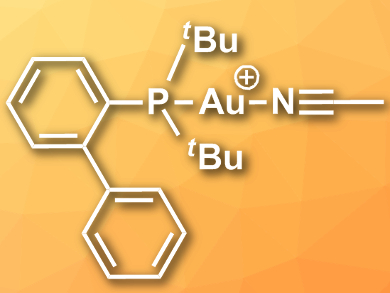
Gold complexes can be used as catalysts for a range of organic transformations. A commonly used gold(I)-based complex is the so-called Echavarren’s catalyst, [Au(NCMe)(PtBu2C6H4Ph-2)][SbF6] (cation pictured).
The synthesis of this catalyst usually involves the oxidation of metallic gold to form tetrachloroauric acid using aqua regia (a mixture of nitric acid and hydrochloric acid), which needs to be removed by distillation. The tetrachloroauric acid is then reduced and converted to a gold-thioether complex and the thioether is replaced by the desired phosphine ligand. In a final step, a silver salt is used to remove the remaining chloride. This step requires very accurate stoichiometry, otherwise, it can leave silver or halide impurities in the final catalyst.
Jason L. Dutton, La Trobe University, Melbourne, Australia, and colleagues have developed a simpler synthesis of Echavarren’s catalyst. The team oxidized gold powder with [NO][BF4] in acetonitrile to directly give the complex [Au(NCMe)2]+, which can be converted to the desired catalyst by adding the phosphine PtBu2(C6H4Ph-2). This method removes the need for aqua regia distillation, saves time, and ensures that the product is free from halide and silver impurities. It is safer than the commonly used synthesis approach and the product is significantly cheaper than commercially bought Echavarren’s catalyst.
- A simple halide and silver-free synthesis of Echavarren’s catalyst directly from gold powder,
Mohammad Albayer, Robert Corbo, Anthony F. Hill, Jason L. Dutton,
Dalton Trans. 2018.
https://doi.org/10.1039/c7dt04494a