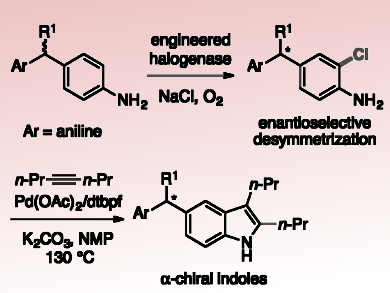
Enzymatic halogenation is a promising tool for inserting halogen substituents due to the high selectivity, mild conditions, and environmentally benign reagents associated with halogenase catalysis. Many flavin-dependent halogenases (FDHs) selectively functionalize C–H bonds on aromatic substrates, often at electronically disfavored positions. Therefore, engineering FDHs with improved stability, expanded substrate scope, and altered regioselectivity is of great interest. To date, however, no examples of enantioselective halogenation catalyzed by FDHs have been reported.
Jared C. Lewis, University of Chicago, Il, USA, and colleagues have engineered the rebeccamycin halogenase (RebH) variant 4-V to halogenate large, biologically active indoles and carbazoles. Recent studies showed that the enzyme also catalyzes remote desymmetrization of methylenedianinelines with high enantioselectivity. This is the first report of an FDH catalyzing an enantioselective halogenation reaction.
The team generated a small group of 4-V variants based on docking simulations of methylenedianiline binding in the enzyme active site. These variants provided improved activity and selectivity on a number of methylenedianiline substrates. This shows how targeted mutagenesis can be used to improve halogenase enantioselectivity in analogy to previous efforts focused on substrate scope and regioselectivity.
The team converted one of the resulting enantioenriched, halogenated methylenedianiline products to 5-substituted indole (pictured) with no loss in enantioselectivity at the α-stereogenic center. According to the researchers, future research will further expand the potential applications of these halogenases in the preparation of useful, bioactive compounds.
- Enantioselective Desymmetrization of Methylenedianilines via Enzyme-Catalyzed Remote Halogenation,
James T. Payne, Paul H. Butkovich, Yifan Gu, Kyle N. Kunze, Hyun June Park, Duo-Sheng Wang, Jared C. Lewis
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.7b09573