Isocyanates are important chemical building blocks which are produced on a scale of millions of tons per year. The industrial synthesis of isocyanates usually requires phosgene, which is highly toxic and very difficult to handle.
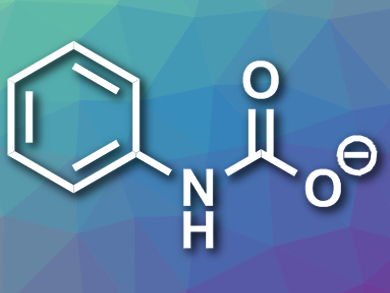
Yiming Ren and Sophie A. L. Rousseaux, University of Toronto, Canada, have developed a synthesis of aryl isocyanates from arylamines that uses CO2 as a C1 source instead of phosgene. The team reacted the arylamines with CO2 and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) to generate carbamic acid intermediates (pictured). These compounds were then dehydrated using dimethyl sulfoxide (DMSO), which was activated with trifluoroacetic anhydride (TFAA), to give the desired isocyanates.
The reaction proceeds under mild conditions in high yields and can convert a wide range of amines to the corresponding isocyanates. The products can be transformed further to give a variety of unsymmetrical ureas and carbamates.
- Metal-Free Synthesis of Unsymmetrical Ureas and Carbamates from CO2 and Amines via Isocyanate Intermediates,
Yiming Ren, Sophie A. L. Rousseaux,
J. Org. Chem. 2017.
https://doi.org/10.1021/acs.joc.7b02905




Hello. It’s a very important development to reduce the use of highly toxic phosgene. I would also like to find safer research, such as the use of sodium cyanate as a source of the ISO to replace aromatic rings, such as salicylic acid or phenol, using simple and safer methods. Greetings to all interested.