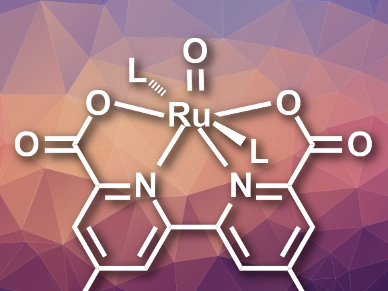
The oxygen evolution reaction (OER) is a challenging part of water oxidation for sustainable fuel production. To improve its efficiency, the catalytic processes during the reaction need to be well understood.
Christophe Copéret, Swiss Federal Institute of Technology (ETH) Zurich, Yulia Pushkar, Purdue University, West Lafayette, IN, USA, and colleagues have prepared a well-defined ruthenium catalyst on an electrode surface. They then used this system to study highly reactive intermediates that are usually difficult to detect. The team synthesized a ruthenium complex of the type [(HNEt3)2][Ru(bdaPhP)(isoq)2] (bdaPhP = 4,4′-bis(4-phosphonophenyl)-[2,2′-bipyridine]-6,6′-dicarboxylate, isoq = isoquinoline). This complex was then grafted to a porous indium tin oxide (ITO) electrode using the phosphate groups as anchors.
The researchers studied the immobilized catalyst using X-ray absorption (XAS) and electron paramagnetic resonance (EPR) spectroscopy. They found a key intermediate featuring a seven-coordinate RuV=O species (pictured). Electrochemical measurements and density functional theory (DFT) calculations led the team to conclude that the nucleophilic attack of water on this intermediate is the rate determining step in OER at low pH values. According to the team, these results could be useful for the design of efficient Ru-based OER catalysts.
- The Key RuV=O Intermediate of Site-Isolated Mononuclear Water Oxidation Catalyst Detected by in Situ X-ray Absorption Spectroscopy,
Dmitry Lebedev, Yuliana Pineda-Galvan, Yuki Tokimaru, Alexey Fedorov, Nicolas Kaeffer, Christophe Copéret, Yulia Pushkar,
J. Am. Chem. Soc. 2017.
https://doi.org/10.1021/jacs.7b11388