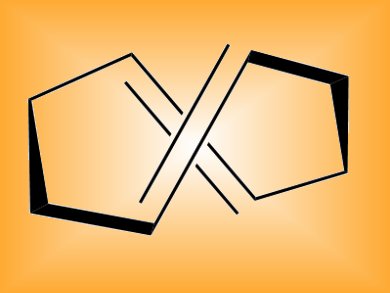
A tool for synthetic organic chemists in the form of (E,E)-1,5-cyclooctadiene could find wide utility in chemical biology and polymer chemistry.
The highly strained molecule (E,E)-1,5-cyclooctadiene performs two fast reactions in so-called “click chemistry” – a reliable approach to synthesis based on “clicking” together simple building blocks. The reagent, known for 40 years but little investigated, undergoes [3+2] cycloadditions with 1,3-dipoles very quickly and the resulting cycloadduct then undergoes inverse-electron-demand Diels-Alder reactions with tetrazines at much higher rates than usual.
It could be used to make novel targeted drug molecules, supramolecular systems for nanotechnology and novel polymeric materials.
- (E,E)-1,5-Cyclooctadiene: a small and fast click-chemistry multitalent
H. Stöckmann, A. A. Neves, H. A. Day, S. Stairs, K. M. Brindle, F. J. Leeper
Chem. Commun. 2011.
DOI: 10.1039/C1CC12161H