The use of CO2 as a feedstock for the chemical industry could help to reduce the dependence on fossil resources and the impact of carbon emissions. Lower olefins such as ethene or propene are needed in large amounts for, e.g., polymer production and are usually produced from fossil fuels. As an alternative, they could theoretically be prepared by the hydrogenation of CO2. However, there a lack of reaction protocols which convert CO2 to lower olefins with high selectivity.
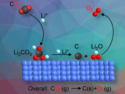
Can Li and colleagues, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, have developed a dual catalyst that can selectively produce lower olefins (between two and four carbon atoms) from CO2. The team prepared a ZnO-ZrO2 binary oxide in aqueous solution and mixed it with a SAPO-34 zeolite in a 1:1 mass ratio to prepare the catalyst. To evaluate the catalyst’s performance, the researchers used a mixture of CO2, H2, and argon at a pressure of 2 MPa and at 380 °C.
The catalyst reached a selectivity for the production of lower olefins of up to 80–90 %. The two catalyst materials work in tandem: CO2 and H2 are activated on the ZnO-ZrO2 component to produce a methanol intermediate, and the C−C bond formation is performed on the zeolite. According to the researchers, the catalyst has a high thermal stability and is resistant to sulfur impurities, which shows its promise for industrial applications.
- Highly Selective Conversion of Carbon Dioxide to Lower Olefins,
Zelong Li, Jijie Wang, Yuanzhi Qu, Hailong Liu, Chizhou Tang, Shu Miao, Zhaochi Feng, Hongyu An, Can Li,
ACS Catal. 2017.
DOI: 10.1021/acscatal.7b03251