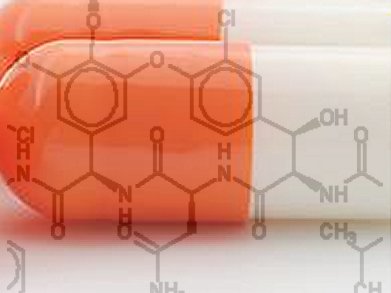
Vancomycin is an antibiotic used only, after treatment with other antibiotics has failed. Vancomycin-resistant bacteria remodel their cell wall precursor peptidoglycan terminus from D-Ala-D-Ala to D-Ala-D-Lac. Chemically, they replace an amide with an ester, reducing the binding of vancomycin to its target 1000-fold and accounting for the loss in antimicrobial activity.
A team of scientists around Dale L. Boger, The Scripps Research Institute, La Jolla, CA, USA, have successfully reengineered vancomycin to kill antibiotic-resistant bacteria. A complementary single atom exchange in the vancomycin core structure (O→NH) to counter the single atom exchange in the cell wall precursors of resistant bacteria (NH→O), reinstates potent antimicrobial activity. Remarkably, the redesigned antibiotic binds to the mutant as well as to the wild type peptidoglycan.
This charts a rational path forward for the development of antibiotics for the treatment of vancomycin-resistant bacterial infections. Reengineered organisms to produce the material or semi-synthetic approaches to the analogue are investigated.
- A Redesigned Vancomycin Engineered for Dual d-Ala-d-Ala and d-Ala-d-Lac Binding Exhibits Potent Antimicrobial Activity Against Vancomycin-Resistant Bacteria,
Jian Xie, Joshua G. Pierce, Robert C. James, Akinori Okano, Dale L. Boger,
J. Am. Chem. Soc. 2011.
DOI: 10.1021/ja207142h