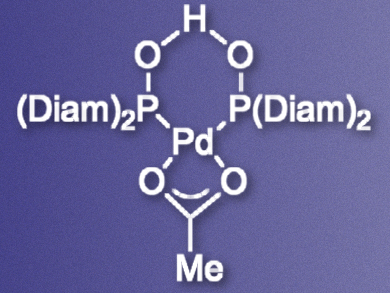
Arylating otherwise inert C–H bonds using palladium catalysts can be a good alternative to traditional cross-coupling reactions. The functionalization of oxazolines, specifically, can provide access to so-called pybox ligands, which are composed of two oxazoline rings connected by a pyridine linker.
Lutz Ackermann, University of Göttingen, Germany, and University of Pavia, Italy, and colleagues have developed a palladium catalyst (pictured) which can promote C–H arylations of oxazolines. The team prepared a bisdiamantyl-substituted secondary phosphine oxide (SPO) ligand and combined it with Pd(OAc)2 in toluene to give the catalyst. The catalyst was then used to promote the reaction of different oxazolines with aryl bromides or chlorides in dimethylacetamide (DMA).
The reaction proceeds in high yields and is tolerant of a range of substrates, including N- and S-heterocycles. The racemization-free reaction conditions allow for the synthesis of chiral pybox ligands, which, in turn, can be used in asymmetric catalysis.
- Secondary Phosphine Oxide Preligands for Palladium-Catalyzed C-H (Hetero)Arylations: Efficient Access to Pybox Ligands,
Debasish Ghorai, Valentin Müller, Helena Keil, Dietmar Stalke, Giuseppe Zanoni, Boryslav A. Tkachenko, Peter R. Schreiner, Lutz Ackermann,
Adv. Synth. Catal. 2017.
DOI: 10.1002/adsc.201700663