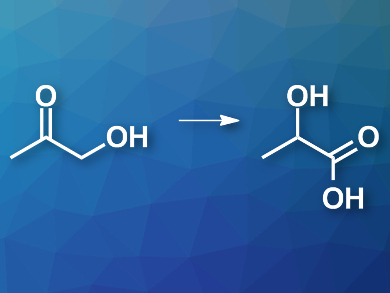
Biomass, e.g, from wood, can be converted into biofuels or renewable chemical feedstocks. Pyrolysis is a common method to convert lignocellulose biomass into useful chemicals. However, the process produces oxidized species, such as acetol (pictured left), which cannot easily be converted into fuels. An alternative is the conversion of acetol to lactic acid (pictured right), which has a range of uses in industry, e.g., in the production of biodegradable polymers.
Shaolong Wan, Xiamen University, China, Yong Wang, Xiamen University and Washington State University, Pullman, USA, and colleagues have developed a one-pot process for converting acetol to lactic acid. The team prepared a catalyst starting from commercial H-Beta zeolite. The zeolite was dealuminated using a HNO3 solution and reacted with tin(II)acetate to give so-called DeAl-Sn-Beta. This modified zeolite was used as a support for gold nanoparticles (NPs), which were loaded onto the material with a sol-immobilization method.
The resulting bifunctional catalyst was able to convert acetol to lactic acid with yields of up to 68 % under relatively mild reaction conditions. The Lewis-acidic tin sites and gold nanoparticles work synergistically to promote the reaction. The proposed mechanism involves an oxidation to pyruvaldehyde catalyzed by the gold NPs, followed by a hydration and a 1,2-hydride shift at the tin sites. The base-free reaction conditions allow the direct formation of lactic acid, as opposed to the lactate salts which are formed using other approaches under alkaline conditions.
- One-Pot Production of Lactic Acid from Acetol over Dealuminated Sn-Beta Supported Gold Catalyst,
Yan Wan, Mengqi Zhuang, Shaopeng Chen, Wenda Hu, Jie Sun, Jingdong Lin, Shaolong Wan, Yong Wang,
ACS Catal. 2017.
DOI: 10.1021/acscatal.7b01499