Vinyllithium (H2C=CHLi) is a reagent used in organic synthesis as a nucleophile. Its heavier silicon analogues, the lithium disilenides, can be used as building blocks in inorganic main group chemistry. There have been several examples of this type of compound, usually with bulky stabilizing substituents. For the germanium analogues (lithium digermenides), in contrast, there had been no fully characterized example in the chemical literature.
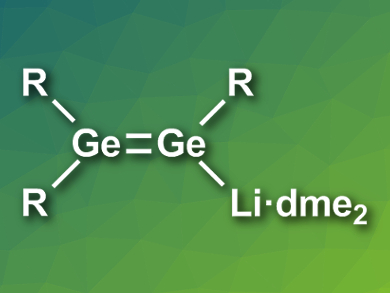
David Scheschkewitz, Saarland University, Saarbrücken, Germany, and colleagues have synthesized the lithium digermenide Tip2Ge=Ge(Tip)Li·dme2 (Tip = 2,4,6-triisopropylphenyl, dme = 1,2-dimethoxyethane, structure pictured). The team started from Tip2GeCl2, which was reduced using lithium powder in dme at –70 °C to give the desired product. The product was characterized using 1H-NMR and UV/Vis spectroscopy, as well as X-ray crystallography.
The digermenide’s reactivity towards chlorosilanes was tested using Me3SiCl and Me2PhSiCl. Both silanes reacted to give silyl-substituted digermenes, which was confirmed by 29Si NMR spectra. According to the researchers, the lithium digermenide could have various applications in the synthesis of low-valent germanium compounds.
- Isolation and Reactivity of a Digerma Analogue of Vinyllithiums: a Lithium Digermenide,
David Nieder, Lukas Klemmer, Yvonne Kaiser, Volker Huch, David Scheschkewitz,
Organometallics 2017.
DOI: 10.1021/acs.organomet.7b00470