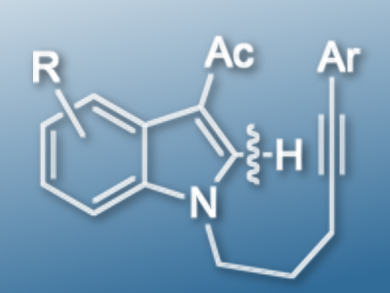
The indole skeleton is often found in bioactive molecules. A common method to synthesize multi-substituted indole derivatives is to install the substituents at the C-2 position via transition-metal-catalyzed C-H activation.
Takanori Shibata, Waseda University, Tokyo, Japan, and colleagues have found that 3-acetyl-N-alkynylindoles (example pictured) undergo a 6-exo-dig cyclization when using [Ir(cod)2]OTf (cod = 1,5-cyclooctadiene) as a catalyst, 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (BINAP) as a ligand, and NaOAc as an additive. However, the same starting material gives unexpected 7-endo-dig cyclization products when using [Rh(cod)2][B((3,5-CF3)C6H3)4] as a catalyst and 2,2′-bis(diphenylphosphino)diphenyl ether (DPEPHOS) as a ligand in the absence of any additives. Both reactions were carried out in chlorobenzene at 135 oC.
Preliminary mechanistic studies show that the presence of a carbonyl directing group is necessary. In Ir catalysis, an oxidative addition was the major pathway for C-H bond activation, instead of a concerted metalation deprotonation although a base was used. An intramolecular hydrometalation is involved in both Ir- and Rh-catalyzed reactions and a trans addition in this step led to the 7-endo products in Rh catalysis.
- Intramolecular C−H Alkenylation of N-Alkynylindoles: Exo and Endo Selective Cyclization According to the Choice of Metal Catalyst,
Takanori Shibata, Takumi Baba, Hideaki Takano, Kyalo Stephen Kanyiva,
Adv. Synth. Catal. 2017, 359, 1849–1853.
DOI: 10.1002/adsc.201700107