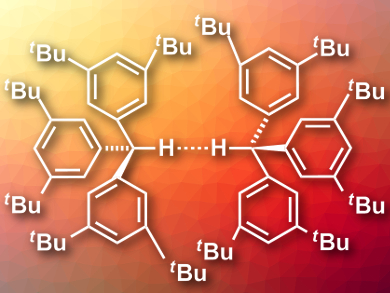
There is a number of examples for molecules with very short intramolecular C–H···H–C distances (down to about 1.6 Å). These compounds often have bowl-like structures that force two hydrogens close together. One would not expect to find such close contacts between separate molecules because of the high amount of energy needed to bring the atoms into proximity.
Peter R. Schreiner, University of Gießen, Germany, and colleagues have synthesized and crystallized tri(3,5-di-tert-butylphenyl)methane (pictured). Using neutron diffraction measurements, the team was able to show that the resulting crystal features the shortest intermolecular C–H···H–C distance known to date with only 1.566(5) Å.
The researchers performed quantum chemical calculations to investigate the bonding situation. They found that crystal packing effects stabilize the close contact, but are not solely responsible for it. In a gas-phase calculation, the distance between hydrogen atoms was still as short as 1.634 Å. The bulky tert-butyl groups in the molecule stabilize the unusually short distance. The groups are highly polarizable, which creates large London dispersion interactions between the molecules that outweigh the energy needed to bring the molecules this close together.
- London Dispersion Enables the Shortest Intermolecular Hydrocarbon H···H Contact,
Sören Rösel, Henrik Quanz, Christian Logemann, Jonathan Becker, Estelle Mossou, Laura Cañadillas-Delgado, Eike Caldeweyher, Stefan Grimme, Peter R. Schreiner,
J. Am. Chem. Soc. 2017.
DOI: 10.1021/jacs.7b01879