Olefins are usually used as coupling partners in transition-metal-catalyzed C–H activation reactions. The olefin norbornene, however, has also been used as a cocatalyst in palladium-catalyzed reactions. Similar reactions had not been discovered in other catalytic systems so far.
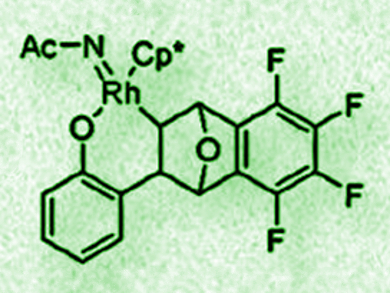
Frank Glorius, University of Münster, Germany, and colleagues have found that 7-oxa-(tetrafluorobenzo)norbornadiene can be used as a cocatalyst in the rhodium(III)-catalyzed intramolecular amide transfer reaction of N–phenoxyacetamides. The researchers propose a nitrenoid (pictured) as the key intermediate. The bicyclic olefin stabilizes one of the intermediates and facilitates the cleavage of the O–N bond.
This C–H bond amidation reaction produces ortho-amide substituted phenols in good yields using 5 mol % [Cp*Rh(MeCN)3](SbF6)2 as the catalyst and one equivalent of the 7-oxanorbornadiene derivative as the cocatalyst in the presence of NaOAc in dichloromethane at room temperature. The method has been used in the late stage diversification of natural products and pharmaceutically active molecules and might be useful for developing other new olefin-cocatalyzed reactions in the future.
- Cp*Rh(III)/Bicyclic Olefin Cocatalyzed C–H Bond Amidation by Intramolecular Amide Transfer,
Xiaoming Wang, Tobias Gensch, Andreas Lerchen, Constantin G. Daniliuc, Frank Glorius,
J. Am. Chem. Soc. 2017.
DOI: 10.1021/jacs.7b02725