Cross-coupling reactions are widely used to form C–C and C–N bonds. While palladium catalysts are still dominantly used, copper catalysts are attracting more attention because they are more abundant, less expensive, and less toxic. Pincer complexes consist of tridentate ligands bind to a metal. They are rarely used in copper chemistry.
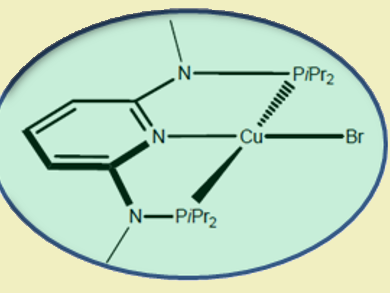
Karl Kirchner, Vienna University of Technology, Vienna, Austria, and colleagues have developed an air-stable, thermally robust, and well-defined copper PNP pincer complex (pictured). They treated CuBr(Me2S) with the pincer ligand PNPMe–iPr, which is based on the 2,6-diaminopyridine scaffold, in dichloromethane at room temperature. The structure of the complex has been determined by single-crystal X-ray diffraction.
The researchers found that this neutral complex was an active catalyst for cross-couplings of (hetero)aryl and aliphatic halides with Grignard reagents, terminal alkynes, and aryl amines, although CuBr(Me2S) itself was poorly active or inactive in those reactions. All reactions were performed in THF with 1 mol % of the catalyst at 50 °C for 16 h. Good to excellent yields were observed in most cases.
- Three Different Reactions, One Catalyst: A Cu(I) PNP Pincer Complex as Catalyst for C−C and C−N Cross-Couplings,
M. Mastalir, E. Pittenauer, B. Stöger, G. Allmaier, K. Kirchner,
Org. Lett. 2017.
DOI: 10.1021/acs.orglett.7b00857