Lithium batteries are the current standard for portable electronics, but due to limited lithium reserves, a need for higher energy densities, and the possible formation of dangerous dendrites that can short-circuit such batteries, alternative materials are sought. Magnesium, for example, could provide cheaper batteries with no risk of dendrite formation and high energy densities. However, there is a lack of suitable non-corrosive halide-free electrolytes. Halides can be oxidized in the battery and damage the metal anode or battery casing.
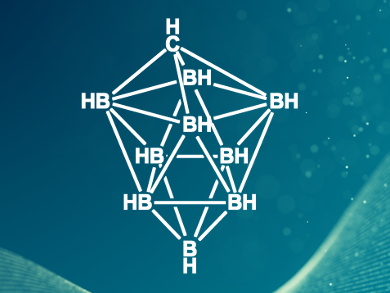
One possible electrolyte is Mg[HCB11H11]2, which contains an icosahedral carborane anion that is electrochemically stable and non-corrosive. However, it is expensive to produce and the synthesis needs toxic and hazardous reagents. Juchen Guo, Vincent Lavallo, and colleagues, University of California, Riverside, USA, have synthesized a 10-vertex carborane anion (pictured) that can be used as an electrolyte for Mg batteries. The team reduced [HNMe3][HCB9H9] with elemental magnesium to give the desired electrolyte Mg[HCB9H9]2. The carborane anion can be prepared easily by the reaction of B10H14 with p-formaldehyde and a subsequent oxidation with iodine.
The developed electrolyte can be produced in a cost-efficient way, has excellent electrochemical stability, is non-corrosive, and is air- and water-stable. According to the researchers, the electrolyte could be useful for commercial, high-density Mg batteries.
- Below the 12-vertex: 10-vertex carborane anions as non-corrosive, halide free, electrolytes for rechargeable Mg batteries,
Scott G. McArthur, Rahul Jay, Linxiao Geng, Juchen Guo, Vincent Lavallo,
Chem. Commun. 2017.
DOI: 10.1039/c7cc01570d