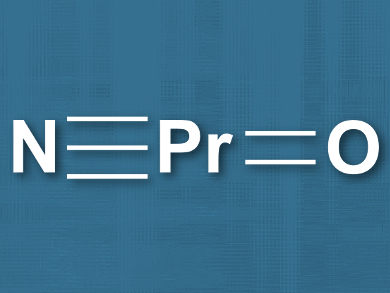Lanthanides, which are important for applications such as catalysis and strong magnets, usually occur in low oxidation states such as +II and +III. The highest known oxidation state had long been +IV. Praseodymium, which has the lowest fifth ionization energy of the lanthanides, has been the most promising candidate for finding higher oxidation states in the series.
Jun Li, Tsinghua University, Beijing, China, Mingfei Zhou, Fudan University, Shanghai, China, and colleagues have synthesized the praseodymium nitride-oxide species NPrO and NPrO–, in which the metal has the oxidation states Pr(V) and Pr(IV), respectively. The team reacted laser-ablated praseodymium atoms and nitric oxide in a solid neon matrix at 4 K. They characterized the products using infrared (IR) spectroscopy and found IR absorptions that can be attributed to NPrO molecules and NPrO– anions.
The team performed quantum chemical calculations to investigate the possible structure of the products and found evidence for a linear structure with a pentavalent Pr (pictured). Natural bond orbital (NBO) analyses were used to confirm the Pr≡N and
Pr=O multiple bonding. According to the researchers, these results indicate that lanthanide compounds with oxidation states higher than +IV might have a richer chemistry than had been known so far.
- The Pentavalent Lanthanide Nitride-Oxides: NPrO and NPrO-Complexes with N–Pr Triple Bonds,
Shu-Xian Hu, Jiwen Jian, Jing Su, Xuan Wu, Jun Li, Mingfei Zhou ,
Chem. Sci. 2017.
DOI: 10.1039/c7sc00710h