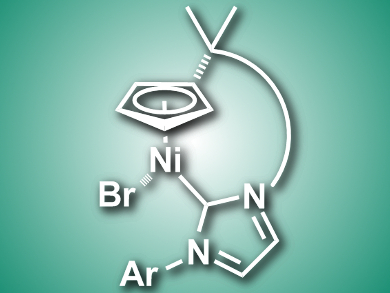
Cyclopentadienyl nickel complexes of the type [Ni(Cp)(X)(NHC)] (Cp = Cyclopentadienyl, X = halogenide, NHC = N-heterocyclic carbene) are useful in catalysis. Chiral variants of such complexes for asymmetric catalysis are not well explored.
Włodzimierz Buchowicz, Warsaw University of Technology, Poland, and colleagues have synthesized an axially chiral half-sandwich nickel complex (pictured) using ring-closing olefin metathesis. The team prepared the diene nickel complex [Ni(η5-C5H4R)(Br)(NHC)] (R = C(CH3)2CH2CH=CH2, NHC = 1-(6-hexenyl)-3-(2,4,6-trimethylphenyl)-imidazol-2-ylidene) and used a ruthenium catalyst to close the nickelacycle.
The complex was charcterized using X-ray crystallography. The researchers found that the Cp–NHC tether forms a helical shape and, thus, the complex has a chiral structure. The product was formed as racemate, but according to the researchers, could have applications in asymmetric synthesis after separation of the enantiomers. The team showed that the racemic mixture is catalytically active in C–C coupling reactions.
- Axially chiral racemic half-sandwich nickel(II) complexes by ring-closing metathesis,
Włodzimierz Buchowicz, Łukasz Banach, Radosław Kamiński, Piotr Buchalski,
Dalton Trans. 2017.
DOI: 10.1039/c6dt04811k