Free oxygen radicals are a byproduct of any metabolism involving O2. Cells usually neutralize radicals since elevated levels result in reactive species such as H2O2 – a state which is called oxidative stress. Oxidative stress can lead to inflammation and DNA damage, which are closely linked to the development of various diseases, e.g. cancer. Oxidative stress is also characteristic for ischemic reperfusion injury: tissues injured by an ischemic event (a restriction in blood supply) show a five-fold increase of H2O2. Unfortunately, there has been no method to detect pathophysiological levels of H2O2 in tissues.
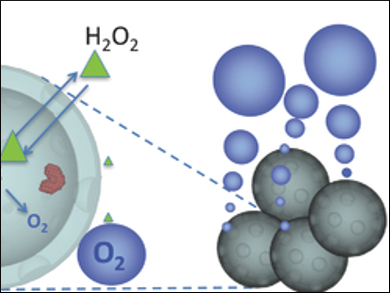
Emilia Olson, University of California, San Diego, USA, and colleagues used intravenously injectable synthetic hollow enzyme-loaded nanospheres (SHELS) to detect sites of oxidative stress in vivo. SHELS are prepared from a nanoporous silica shell and catalase, an enzyme that converts H2O2 to water and O2. The resulting catalase-SHELS (catSHELS) enclose the catalase, while still allowing the contact with H2O2 to produce O2.
The catSHELS were tested in vivo in rats with an induced ischemic injury. After injection of the catSHELS, an increased ultrasound signal was detected due to O2 microbubbles. These are the result of the catalase activity and the elevated H2O2 levels after the ischemic event. According to the researchers, their approach could allow using H2O2 elevation as a biomarker for early disease detection.
- Ultrasound Detection of Regional Oxidative Stress in Deep Tissues Using Novel Enzyme Loaded Nanoparticles,
Emilia S. Olson, Inanc Ortac, Christopher Malone, Sadik Esener, Robert Mattrey,
Adv. Healthcare Mater. 2017.
DOI: 10.1002/adhm.201601163