A wide variety of different ligands can be used to tune the properties of transition metal complexes, e.g., for catalysis. Ligands generally donate electron density to the metal center and are most often neutral or anionic. Cationic ligands, in contrast, are not nearly as common.
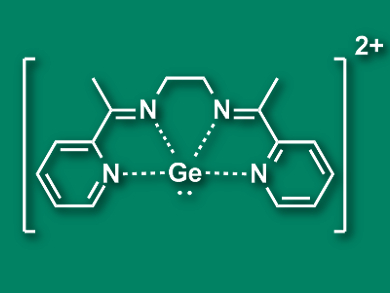
Ravindra K. Raut and Moumita Majumdar, Indian Institute of Science Education and Research, Pune, have prepared a dicationic germanium complex (pictured) which can bind to the transition metal centers Ag(I) and Au(I). This is the first example of a cationic group 14 ligand in a transition metal complex. The team synthesized the triflate salt of the Ge complex by combining a solution of GeCl2·dioxane and the ligand 2,7-bis(2-pyridyl)-3,6-diazaocta-2,6-diene (L) in CH2Cl2 with trimethylsilyl triflate (TMSOTf).
The [GeL]2+ complex (pictured), despite its positive charge, can bind to Ag+ and Au+ ions and form nearly linear complexes of the type [LGe-M-GeL][OTf]5. The researchers used density functional theory (DFT) calculation to understand the bonding situation in the resulting complexes. They found a σ-type donation of electrons from the lone pair at Ge to the transition metal and a π-type back-donation from the transition metal towards the germanium. The π-interaction compensates for the weak donor characteristics of the cationic ligand and strengthens the bond.
- Direct coordination of a germanium(II) dicationic center to transition metals,
Ravindra K. Raut, Moumita Majumdar,
Chem. Commun. 2017.
DOI: 10.1039/c6cc09525a