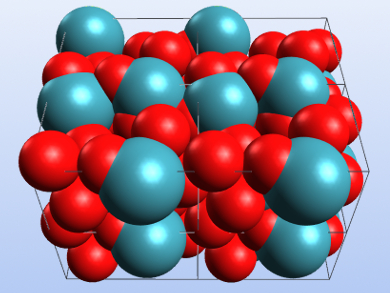
Xenon trioxide (XeO3) is extremely explosive as well as shock-, temperature-, and light-sensitive. It can be synthesized by the hydrolysis of XeF4 and crystallized by slow evaporation of its aqueous solution. XeO3 obtained in this way has been characterized using X-ray crystallography and was found to form an orthorhombic phase (α-XeO3) with the space group P212121.
James T. Goettel and Gary J. Schrobilgen, McMaster University, Hamilton, Ontario, Canada, have reinvestigated the solid-state structure of the compound and found two new phases by modifying the crystallization conditions. The team obtained α-XeO3 by slowly evaporating a solution of XeO3 in 48 % HF solution. β-XeO3 was synthesized by slow water evaporation from aqueous XeO3 solutions. Evaporation of a solution of XeO3 in propionitrile (H3CCH2CN) gave crystals of γ-XeO3.
The researchers characterized the obtained phases by single-crystal X-ray crystallography and Raman spectroscopy. They obtained a more precise structure of α-XeO3 that was in agreement with previously reported data. The β– and γ-phases are rhombohedral with the point groups R3 and R3c, respectively. According to the team, all three structures feature Xe=O—Xe bridges resulting from the simultaneous donor and acceptor properties of XeO3.
- Solid-State Structures of XeO3,
James T. Goettel, Gary J. Schrobilgen,
Inorg. Chem. 2016.
DOI: 10.1021/acs.inorgchem.6b02371