Hydroxylated silicon surfaces are most commonly functionalized using the hydrolytic condensation of organotrialkoxysilanes. While this method is widespread, it suffers from long reaction times and needs elevated temperature. Additionally, organotrialkoxy-silanes can undergo cross-linking, which may block the pores in porous silicon materials.
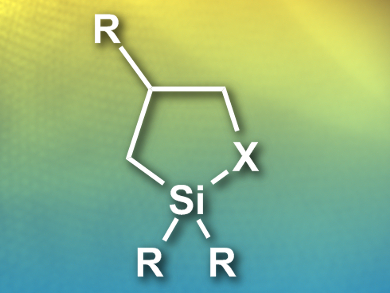
Michael J. Sailor, University of California, San Diego, USA, and colleagues have developed a simple method for surface functionalization by replacing organotrialkoxy-silanes with heterocyclic silanes. In a ring-opening click reaction, five-membered heterocyclic compounds with silicon-sulfur or silicon-nitrogen motifs (example pictured) readily react with the surface hydroxyl groups present on porous silicon materials.
The reaction proceeds at room temperature without the formation of any byproducts. The researchers found significantly reduced reaction times and better coverage compared to the synthetic route using organotrialkoxysilanes. In addition, the pore structure in the materials was retained, and a one-pot tandem functionalization with succinic anhydride is also possible.
- Facile Surface Modification of Hydroxylated Silicon Nanostructures Using Heterocyclic Silanes,
Dokyoung Kim, Jonathan M. Zuidema, Jinyoung Kang, Youlin Pan, Lianbin Wu, David Warther, Barry Arkles, Michael J. Sailor,
J. Am. Chem. Soc. 2016.
DOI: 10.1021/jacs.6b08614

![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

