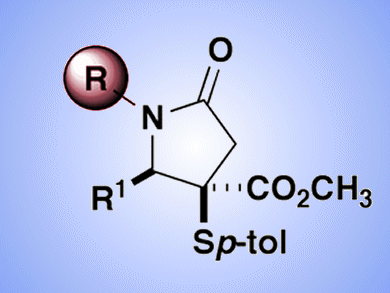
Five-membered ring lactams, γ-lactams, are important structural motifs in biologically active natural products, but γ-lactams that are substituted at the 1-position (nitrogen) are less common in drug leads. A survey of γ-lactams of in preclinical and clinical development showed a nearly two-to-one ratio of N–H lactams (unsubstituted) over their substituted counterparts. This is perhaps due to synthetic limitations.
Jared Shaw and co-workers, University of California – Davis, USA, have reported a multicomponent methodology for the synthesis of unsubstituted γ-lactams and their subsequent N-functionalization. By including ammonium acetate as the starting material in a previously reported four component reaction (4CR), the team demonstrated a range of N–H lactam structures could be produced. These substrates could then be functionalized at nitrogen through acylation or arylation to provide structural diversity unavailable through the original 4CR.
- Ammonia synthons for the multicomponent assembly of complex γ-lactams
D. Q. Tan, K. S. Martin, J. C. Fettinger, J. T. Shaw,
Proc. Natl. Acad. Sci. 2011.
DOI: 10.1073/pnas.1015261108