Genome-wide Assay System Detects Cisplatin Action Sites
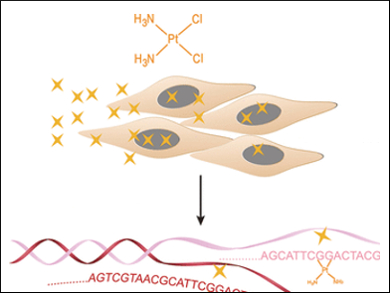
Cisplatin is one of the most widely used agents in cancer chemotherapy. Its mode of action is cross-linking of the DNA, which can kill cells. But which part of the genome is more affected, and which is less affected? A Chinese team of scientists has now set up a universal, genome-wide assay system to detect the specific cisplatin action sites, In the journal Angewandte Chemie the scientists introduce their system and report initial results, which support the notion that the mitochondrial genome is one of cisplatin’s main targets.
Cancer cells are known to be extraordinarily active cells with their DNA replicating at a fast rate. Thus many anticancer drugs are designed to target DNA, and cisplatin is one most effective DNA-damaging agents. Its mode of action is relatively simple: The platinum atom binds to two neighboring DNA bases called guanines and cross-links them, thus widening the DNA duplex structure. If the DNA repair machinery cannot excise and refill all the damaged positions, the cell reprograms itself to induce apoptosis and cell death.
Cisplatin-seq
Although the cisplatin action mechanism is quite clear, there has been only indirect evidence of the exact part of the genome preferentially targeted by cisplatin. In an interdisciplinary collaboration, Chengqi Yi from Peking University, Beijing, China, and Chuan He from Peking University, University of Chicago and Howard Hughes Medical Institute, Chicago, USA, have developed a universal test system called “cisplatin-seq” to identify specifically the genomic parts preferentially attacked by cisplatin.
Key in their test system is the enrichment of cisplatin-affected sites by taking advantage of a specific DNA-binding protein, which was called HMGB1. A segment of this protein (its domain A) was used to enrich the fragments containing the cisplatin- modified DNA and subject them to high-throughput sequencing. The goal was to identify the exact bases where cisplatin had added. “Owing to the ability of cisplatin-DNA adducts to stall DNA synthesis, cisplatin cross-linking sites can be identified at base resolution through the genome,” Yi, He, and their colleagues wrote. Or to put it differently, the sequencing stops exactly at the site where the cross-linking has occurred.
Mitochondrial DNA a Major Target
The big advantage of cisplatin-seq is the detection of cisplatin-DNA adducts genome-wide. Thus the scientists could verify the previous assumption that it is the mitochondrial DNA that is especially sensitive to cisplatin action. “Mitochondrial DNA, which is devoid of histone proteins or NER (one of the DNA repair systems), was found to be a major target of cisplatin,” they said, adding: “…while for DNA in the nucleus of cancer cells, binding of proteins to DNA largely contributes to the accumulative pattern of cisplatin-DNA adducts.”
Further applications of cisplatin-seq could include, for example, the profiling of cisplatin-DNA adducts under different dosages of cisplatin treatment. It would be a highly interesting further step in cancer therapy and analytics.
- Base-Resolution Analysis of Cisplatin-DNA Adducts at the Genome Scale,
Xiaoting Shu, Xushen Xiong, Jinghui Song, Chuan He, Chengqi Yi,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201607380