Iron-catalyzed cross-coupling reactions have made great strides against more conventional palladium-mediated processes, due to the favorable cost, availability, and environmental properties of iron. However, the identity of iron intermediates involved in the iron cross-coupling catalytic cycle has remained unclear.
Konrad Koszinowski and Tobias Parchomyk, Georg-August-Universität Göttingen, Germany, have performed an in-depth study showing the presence of in situ-formed organoferrate ions in a model iron-catalyzed cross-coupling reaction. The researchers used electrospray ionization mass spectrometry (ESI-MS) to identify the iron species in solution. The model reaction used was that of PhMgCl and iPrCl in the presence of Fe(acac)3 (acac = acetylacetone).
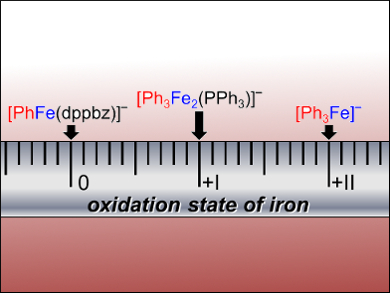
The researchers successfully identified phenylferrate species in the reaction using this method. The 1,2-bis(diphenylphosphino)benzene (dppbz) ligand proved to be a good stabilizer of low-valent ferrate species. The reasoning behind the complex mechanism of iron-catalyzed cross coupling was also revealed: Iron species in different oxidation states react with the iPrCl substrate. Reductive elimination of the key intermediate [Ph3FeiPr] forms the cross-coupled product PhiPr. Gas-phase fragmentation experiments show that the reactivity of phenylferrates in different oxidation states can differ strongly.
These results improve the mechanistic understanding of iron-catalyzed cross-coupling reactions and show that organoferrate anions can act as catalytic intermediates.
- Ate Complexes in Iron-Catalyzed Cross-Coupling Reactions,
Tobias Parchomyk, Konrad Koszinowski,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201603574