It was over two centuries ago that Coulomb first published his theory that like charges repel each other. However, recently, the concept of anti-electrostatic hydrogen bonding laid the theoretical groundwork for the existence of anion–anion dimers. Coulomb repulsion is not the only challenge to the formation of these dimers in solution: Solvation can also separate the anions.
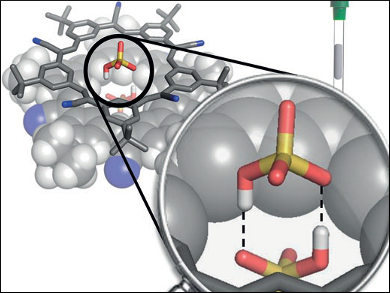
Amar H. Flood and colleagues, Indiana University, Bloomington, USA, could show that within a so-called cyanostar macrocycle pair (C5-symmetric macrocycles), two hydrogen-bonding HSO4− ions can form an anion–anion dimer (pictured). The complex persists in solution. 1H NMR spectroscopy results provide evidence for the bisulfate dimer’s OH⋅⋅⋅O hydrogen bonding with a peak at 13.75 ppm. The dimers in this bisulfate homodimer stabilize each other with self-complementary hydrogen bonds by encapsulation inside a pair of cyanostar macrocycles
The results demonstrate the effectiveness of a supramolecular strategy for the stabilization of reactive species in “hostile” environments, and represent the first conclusive observation of a hydrogen-bonded pair of anions.
- Anions Stabilize Each Other inside Macrocyclic Hosts,
Elisabeth M. Fatila, Eric B. Twum, Arkajyoti Sengupta, Maren Pink, Jonathan A. Karty, Krishnan Raghavachari, Amar H. Flood,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201608118