Lunch out of the microwave usually doesn’t taste nearly as good as a meal made in a conventional oven. This difference in quality is reversed for graphitic carbon nitride, a catalyst used for generating hydrogen from sunlight. Treatment of a precursor material with microwaves delivers a significantly more crystalline product than conventional thermolysis in an oven. As scientists report in the journal Angewandte Chemie, a catalyst produced in this way is significantly more effective in the generation of hydrogen.
Graphitic Carbon Nitrid: Photocatalyst for Water Splitting
Photocatalytic water splitting, in which sunlight is used to split water into hydrogen and oxygen is an environmentally friendly method for generating hydrogen for various applications, including fuel cells. However, the success of this method depends on the efficiency of the photocatalyst.
Graphitic carbon nitride (g-C3N3) is a highly promising candidate. This material consists of six-membered rings made of carbon and nitrogen atoms. Groups of three rings are fused together at the edges (triazine groups), and these are bound into a two-dimensional layer by means of an additional nitrogen atom. This layered structure of six-membered rings is reminiscent of graphite. Unlike graphite, however, g-C3N3 is a semiconductor.
The more perfect the crystal lattice of the g-C3N3, the more effectively it can capture energy form the sun and convert it into hydrogen, a transportable and useful energy carrier; defects in the crystal lattice compromise this efficiency. Researchers at Anhui University, Hefei, China, Harbin Normal University, Harbin, China, and the Georgia Institute of Technology, Atlanta, USA, have now developed a new method for producing g-C3N3 that results in a highly crystalline product with remarkably few defects.
New Method for Producing Graphitic Carbon Nitrid
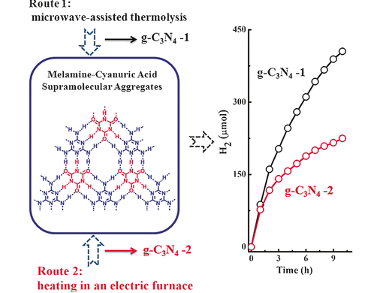
The team led by Yupeng Yuan and Zhiqun Lin first produced an aggregate made of two different starting materials in a solvent. These materials, melamine and cyanuric acid, consist of six-membered rings of carbon and nitrogen atoms. The particular side-groups on these molecules allow them to form hydrogen bridge bonds to each other. This results in extensive two-dimensional aggregates in which the two molecules alternate. This puts the building blocks into the right structure for subsequent bonding of the rings to form g-C3N3, which reduces the likelihood of defects in the lattice.
Instead of bonding the aggregates together by heating in an electric oven (thermolysis), the researchers elected to treat them with microwaves. This process is not only much faster (16 minutes was sufficient), it also produced a material that has twice the photocatalytic activity in the generation of hydrogen. This results from the significantly improved crystallinity that is obtained because the microwave radiation excites the nitrogen-rich molecules, causing intense rotation, friction, and collision.
- A Rapid Microwave-Assisted Thermolysis Route to Highly Crystalline Carbon Nitrides for Efficient Hydrogen Generation,
Yufei Guo, Jing Li, Yupeng Yuan, Lu Li, Mingyi Zhang, Chenyan Zhou, Zhiqun Lin,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201608453




Nice