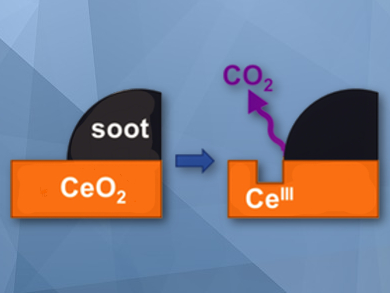
Diesel particulate filters (DPFs) are a valuable technology for soot elimination from diesel engine exhaust. The combination of DPFs with an active catalyst allows the regeneration of the filter by combustion of collected carbon soot at relatively low temperatures. Ceria (CeO2) is one of the most valuable catalysts for the oxidation of carbon soot and the development of robust ceria-based catalysts has considerably progressed over the last years. However, their mode of operation has not been fully understood.
Jordi Llorca, Universitat Politècnica de Catalunya, Barcelona, Spain, and colleagues used near-ambient pressure X-ray photoelectron spectroscopy (XPS) for the first time to investigate the carbon soot oxidation reaction under conditions which are close to the real application. The experiments were performed at the ALBA Synchrotron Light Source near Barcelona. The technique allowed a direct measurement of the cerium’s redox chemistry.
According to the researchers, ceria-based catalysts oxidize carbon through two cooperative routes; one involves the formation of oxygen vacancies at the surface of the ceria and is strongly dependent on the ceria–carbon contact, and the other is based on the generation of active superoxide species from reduced ceria, which act as the true oxidant. The results show that the two routes operate simultaneously and mutually reinforce each other.
- Ambient Pressure Photoemission Spectroscopy Reveals the Mechanism of Carbon Soot Oxidation in Ceria-Based Catalysts,
Lluís Soler, Albert Casanovas, Carlos Escudero, Virginia Pérez-Dieste, Eleonora Aneggi, Alessandro Trovarelli, Jordi Llorca,
ChemCatChem 2016, 8, 2748–2751.
DOI: 10.1002/cctc.201600615