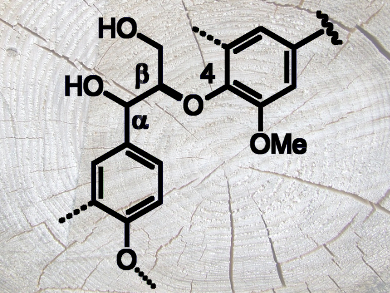
Paper is one of the most widely used industrial products. However, the main byproduct of the paper fabrication process, the polyphenolic macromolecule lignin, is not often used in industrial processes due to its structural heterogeneity. The most common native substructure in lignin is the so-called β-O-4 unit (roughly 50–65 %, pictured). It could be a valuable target in the cleavage of the lignin macromolecules during biorefinery processes. However, the reactivity of β-O-4 units remains poorly understood.
Joseph J. Bozell, University of Tennessee, Knoxville, USA, and colleagues have developed an enantioselective synthesis of (R,R)-, (S,S)-, (R,S)-, and (S,R)-β-O-4 lignin dimer models. The team used an Evans chiral aldol reaction and a subsequent Mitsunobu inversion with excellent ee (> 99 %) and high overall yields. The reaction could be scaled up to provide multigram quantities. The researchers extended the method to perform the first synthesis of an enantiomerically pure lignin trimer.
These model compounds could help to understand the influence of stereochemistry on the chemical, enzymatic, and microbial degradation of lignin.
- Enantioselective Syntheses of Lignin Models: An Efficient Synthesis of β-O-4 Dimers and Trimers by Using the Evans Chiral Auxiliary,
Costyl N. Njiojob, Joseph J. Bozell, Brian K. Long, Thomas Elder, Rebecca E. Key, William T. Hartwig,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201601592