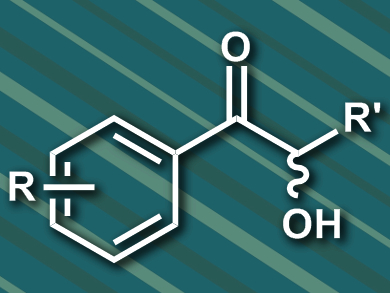
2-Hydroxy ketones, also known as acyloins, are valuable chiral compounds in asymmetric synthesis. Benzoin reactions, a type of catalytic asymmetric C–C bond formation, are used to form 2-hydroxy ketones. In nature, thiamine diphosphate (ThDP)-dependent enzymes can catalyze the formation of 2-hydroxy ketones. In the cross-benzoin reaction of an aliphatic and an aromatic aldehyde, several different products can be formed (example pictured). Certain isomers of these products are difficult to access in a regio- and stereoselective manner.
Michael Müller, University of Freiburg, Germany, and colleagues have found a selective catalytic asymmetric pathway to each 2-hydroxy ketone isomer among all possible products using divergent catalysis. They used ThDP-dependent enzymes as catalysts for an aliphatic-aromatic cross-benzoin reaction, and investigated the scope and limitations of their approach.
The researchers used a set of two R-selective and two S-selective ThDP-dependent enzymes. They achieved the divergent selective synthesis of both R– and S-isomers of 2-hydroxypropiophenone and phenylacetylcarbinol, as well as derivatives thereof. By choosing the appropriate enzyme, the team was able to form the desired product in a regio- and stereoselective manner.
This divergent catalytic system can, in principle, provide access to every desired 2-hydroxy ketone isomer selectively, even the thermodynamically less stable one, under kinetically controlled conditions.
- Regio- and Stereoselective Aliphatic-Aromatic Cross-Benzoin Reaction: Enzymatic Divergent Catalysis,
Maryam Beigi, Ekaterina Gauchenova, Lydia Walter, Simon Waltzer, Fabrizio Bonina, Thomas Stillger, Dörte Rother, Martina Pohl, Michael Müller,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201602084