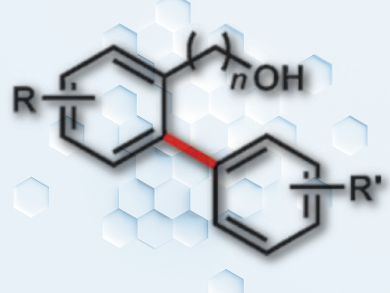
Aromatic C–H activation has become a preferred strategy for synthesizing core structures for pharmaceuticals and natural products. This is due to its high atom economy and its independence from prior functionalization. To render this strategy selective, directing groups are often required.
Eric Ferreira and colleagues, University of Georgia, Athens, USA, have developed a selective route to biaryls through ortho-arylation of benzyl alcohols. The team used quinolinyl acetal (QuA) as a cleavable and recyclable directing group that readily attaches to alcohols. The reactions use N-acetyl isoleucine ligand-accelerated palladium(II)-catalyzed conditions.
QuA can be used with a variety of arylboronic ester coupling partners, including those with ortho substituents, in yields up to 89 %. The incorporation of a quinolinyl group into the directing group helps to temper its Lewis acidity, and it can be readily attached to and removed from a variety of benzylic alcohol substrates.
- Palladium(II)-Catalyzed ortho-Arylation of Aromatic Alcohols with a Readily Attachable and Cleavable Molecular Scaffold,
Qiankun Li, Brian J. Knight, Eric M. Ferreira,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201602844