Interconversion between NH (amido) and NH2 (amine) ligands in transition-metal hydrogen-transfer catalysts can facilitate effective H+ and H– delivery between alcohols and carbonyl compounds. For example, half-sandwich iridium complexes with a C–N chelating benzylic amine moiety can act as highly efficient hydrogen transfer catalysts.
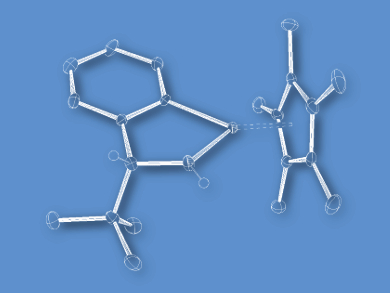
Yasuhiro Sato, Yoshihito Kayaki, and Takao Ikariya, Tokyo Institute of Technology, Japan, have developed chiral azairidacycles derived from optically active primary benzylic amines. Cyclometalation of O-silylated 2-amino-2-phenylethanols and subsequent deprotonation selectively afforded amido-bridged dinuclear complexes. In contrast, more sterically hindered amines were solely transformed into the corresponding mononuclear amidoiridium complexes (example pictured).
In contrast to the dinuclear complexes, the isolated mononuclear complexes serve as an active catalyst species, leading to improved catalytic performance in the asymmetric transfer hydrogenation of acetophenone with a selectivity up to 81 % ee.
- Comparative Study of Bifunctional Mononuclear and Dinuclear Amidoiridium Complexes with Chiral C-N Chelating Ligands for the Asymmetric Transfer Hydrogenation of Ketones,
Yasuhiro Sato, Yoshihito Kayaki, Takao Ikariya,
Chem. Asian J. 2016.
DOI: 10.1002/asia.201600955