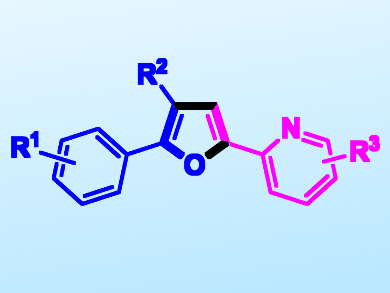
Furans are very diverse and fundamental building blocks in synthetic chemistry. They can be found in a wide range of natural products and in several pharmaceuticals. There are common synthetic methods used to make furans, such as the Paar–Knorr synthesis or the Feist–Benary cyclocondensation. These methods, however, are often ill-suited for synthesizing substituted derivatives. This is due to the often limited availability of functionalized precursors and the harsh conditions necessary.
Qiang Liu, Lanzhou University, Gansu, China, and colleagues have developed a free-radical, one-pot, iridium-catalyzed method for the synthesis of furans under visible-light irradiation. The process allows the combination of styrenes and α-cloro-alkyl ketone radicals to give a wide array of polysubstituted furans (pictured). The team uses fac-Ir(ppy)3 (ppy = 2-phenylpyridin) as a photocatalyst and K2S2O8 as an oxidant. The proposed mechanism is a radical addition/oxidation pathway.
The process can use a wide variety of cheap starting materials and has good functional group tolerance. According to the researchers, the results offer a framework for the further development of related visible-light photocatalysis methods.
- Domino Radical Addition/Oxidation Sequence via Photocatalysis: One-Pot Synthesis of Polysubstituted Furans from α-Chloro-Alkyl Ketones and Styrenes,
Qiang Liu, Shuang Wang, Wen-Liang Jia, Lin Wang, Li-Zhu Wu,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201602053