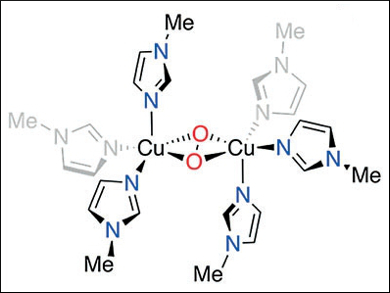
Tyrosinase enzymes (Ty), found in nearly all aerobic life forms, oxidize substrates through a binuclear copper active site that activates dioxygen. The resulting side-on peroxide CuII species converts tyrosine to melanins for pigment formation in animals and plants.
Daniel P. Stack, Stanford University, CA, USA, and colleagues have shown that the simplest synthetic active-site analogue (pictured) can be formed by a self-assembly process. Substituted imidazoles, CuI, and O2 organize in solution at a temperature of –145 °C. The substrate reactivity is similar to that of Ty but apparently proceeds through a mechanism not postulated for the biological oxidation. The researchers charaterized a high-valent bis-CuIII bis-oxide intermediate.
While the assembly and CuIII identification are surprising, the stability and facile reactivity reveal a detail of Ty chemistry: The tertiary protein structure may not be needed, e.g., to impose structural constraints or induce function, since the active-site core is innately reactive.
- Simplest Monodentate Imidazole Stabilization of the oxy-Tyrosinase Cu2O2 Core: Phenolate Hydroxylation through a CuIII Intermediate,
Linus Chiang, William Keown, Cooper Citek, Erik C. Wasinger, T. Daniel P. Stack,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201605159