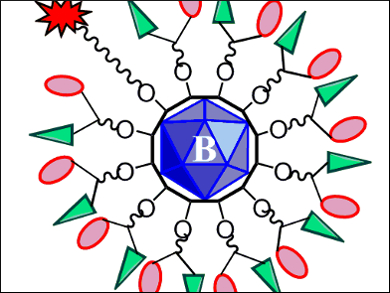
The ultimate goal of cancer therapy is to be highly tumor-targeted, as noninvasive as possible, and to have few side effects. This is theoretically realized by drugs which are so good that they work in low doses and are able to selectively target cancer cells over healthy ones.
M. Frederick Hawthorne, University of Missouri, Columbia, USA, and colleagues have made a multifunctional drug-delivery system with such characteristics. The team used a closomer, a compound based on a closo-[B12OH12]2– polyhedral borane, as a scaffold. The twelve available sites of this closomer are loaded with different groups: One has a fluorescein attachment that allows for real-time fluorescent tracing. The other eleven are linked to glucosamine and chlorambucil (pictured).
Glucosamine is a GLUT-1 receptor target, which makes the drug a good “hunter” of cancer cells and improves water-solubility and bioavailability. Chlorambucil is a therapeutic agent against chronic lymphocytic leukemia, amongst other cancers. The team also made a similar compound without the glucosamine attachment.
Having eleven active drug-carrying sites in one molecule increases drug efficiency while lowering dosage levels. The researchers tested the cytotoxic effect of the compounds on Jurkat cells, a human T-cell leukemia cell line. The fully-loaded drug compound showed enhanced cytotoxic activity compared to chlorambucil, whereas the version without glucosamine showed similar activity to chlorambucil. This shows that the ‘cancer tracker’ glucosamine part is responsible for the increase in activity.
- A Trimodal Closomer Drug-Delivery System Tailored with Tracing and Targeting Capabilities,
Saurav J. Sarma, Aslam A. Khan, Lalit N. Goswami, Satish S. Jalisatgi, M. Frederick Hawthorne,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201602413