Obtaining Rare Earths
Scientists in the U.S. have provided a new understanding of the structure and crystal properties of the main mineral source of rare earth metals, bastnäsites. They are fluoro-carbonate minerals which contain ytterbium, lanthanum, and cerium, among other elements. The researchers used powder X-ray diffraction (XRD) and density functional theory (DFT) to reveal details of the minerals’ structure and interfacial energy. The work could help in the design of new reagents for selective binding to mineral interfaces and could improve the recovery of rare metals by froth flotation, which is the major stage of ore beneficiation. Increasing flotation concentrate grades makes the subsequent leaching and rare earth separations more efficient and economic.
Rare earth elements are increasingly important in modern technology – for electronics, catalysis, possible future quantum devices, and especially for clean energy applications like wind and solar energy, energy-efficient lighting, and electric vehicles. For example, neodymium and praseodymium are used in strong permanent magnets, lanthanum and cerium are used in batteries, metal alloys, petroleum refining, and catalysis, and ytterbium is a common material in phosphors for displays and in high-tech ceramics.
These elements, which are defined as the fifteen lanthanides, as well as scandium and yttrium, are commonly found in the same ores. Despite what their name suggests, they are not actually rare, but they are difficult and costly to refine. As such, it is crucial that scientists and technologists optimize ore beneficiation to provide an enriched feedstock for the subsequent efficient extraction of these elements from the mined mineral ores in which they are found.
Geopolitical Minerals
The research to diversify the supplies of rare earths is particularly crucial as many of the sources for these ores are in parts of the world that can be somewhat geopolitically inaccessible. For instance, China has historically been a rich source of rare earth metals, supplying approximately 90 % of the global rare earth supply. “Reliance on this single supplier creates considerable supply risk for rare earths, rendering clean-energy technologies vulnerable. It is, therefore, desirable to develop new sources of supply,” explains Vyacheslav Bryantsev, Oak Ridge National Laboratory, TN, USA.
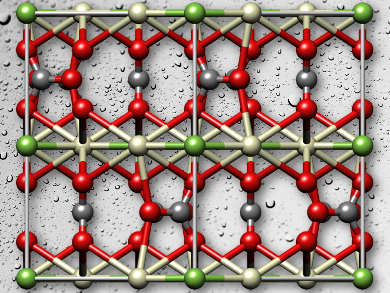
Bastnäsite, also known as bastnaesite, exists in various forms, the most common, bastnäsite-(Ce), has the formula (Ce, La)CO3F, bastnäsite-(La) is (La, Ce)CO3F, and bastnäsite-(Y) is (Y, Ce)CO3F. There are analogous hydroxylbastnäsites, in which the fluoride ions are replaced by hydroxyl ions. The minerals are found in deposits in continental Europe, Russia, Asia, Australia, and the Americas. Large known deposits of bastnäsite are in Bayan Obo, China, and Mountain Pass, CA, USA. For other minerals, there are various methods of mineral extraction, including gravity, magnetic, and electrostatic separations, but for bastnäsite, “froth flotation” is the most widely used technique, because it can be tuned for the specific mineral being processed.
Better Flotation Agents
Vyacheslav S. Bryantsev, together with Alexandra Navrotsky, University of California, Davis, USA, and colleagues explain how the design of improved flotation agents that selectively bind to the mineral surface could be facilitated by an improved understanding of the fundamental bulk and surface properties of bastnäsite itself.
A collector molecule, such as a fatty acid, can be used to latch on to the rare earth metal ions and form a froth that can be skimmed off from the mixture, leaving behind solution and residue containing the bulk of the unwanted minerals. Unfortunately, fatty acids are not actually the most effective nor the most selective of agents one might use in froth flotation. The whole process of finding better agents has been by trial and error so far. The researchers hoped to remedy that situation and use a deliberate design approach to determine the physicochemical characteristics of the structure and surfaces of the crystalline minerals, which might allow them to design much more efficient and effective collector agents.
One important discovery from their powder XRD, DFT, and water adsorption calorimetry studies on bastnäsite is that the structure of bastnäsite-(La) which is reported in the Inorganic Crystal Structure Database is far less stable than the isomorphic bastnäsite-(Ce). This implies that the structure ought to be determined again more definitively. It also has implications for how organic ligands used as collector agents might interact with the mineral surfaces. “The results of this study lay an important foundation for the design of improved flotation agents that can discriminate bastnäsite from the other carbonate materials based on selective binding to the most exposed mineral surface,” the team reports.
Process Analysis
Mineral processing expert Saeed Chehreh Chelgani of the mining department at the University of Michigan, Ann Arbor, USA, points out that, in general, XRD analysis deals with the crystal and matrix and is only semi-quantitative in the context of the surface chemistry of interest here.
“There is a long story from matrix to surface,” he says and “XRD analysis provides information about the mineralogy, not chemistry.” He says that X-ray fluorescence might be a useful additional tool to assist in this research, but also suggests that the science might move forward more rapidly with the use of time-of-flight secondary-ion mass spectrometry (TOF-SIMS) or Fourier-transform infrared (FTIR) spectroscopy.
- Crystal Structures, Surface Stability, and Water Adsorption Energies of La-Bastnäsite via Density Functional Theory and Experimental Studies,
Sriram Goverapet Srinivasan, Radha Shivaramaiah, Paul R. C. Kent, Andrew G. Stack, Alexandra Navrotsky, Richard Riman, Andre Anderko, Vyacheslav S. Bryantsev,
J. Phys. Chem. C 2016.
DOI: 10.1021/acs.jpcc.6b04747