Artificial photosynthesis is one of the most promising methods for enabling the widespread use of renewable energy. In particular, water oxidation (WO) is considered a bottleneck in the process of removing protons and electrons from water to generate solar fuels, such as H2, CO, HCOOH, MeOH, and so on.
In order to develop a commercially useful system, low-cost and highly efficient water oxidation catalysts are required. It is also important to study the inhibition pathways of water oxidation catalysis for the development of durable systems. Copper phthalocyanines are widely used dyes, and are thus readily available and inexpensive, making them an ideal candidate to meet global energy needs if they can be designed to have high catalytic performance.
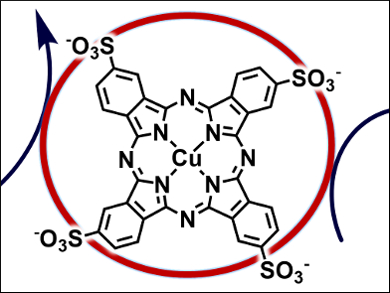
Alexander Rene Parent, Kyushu University, Fukuoka, Japan, and North Dakota State University, Fargo, ND, USA, Ken Sakai, Kyushu University, and colleagues have investigated the catalytic WO activity of copper(II) tetrasulphonatophthalocyanine (pictured) in the [Ru(bpy)3]2+/S2O82– photochemical system. The catalyst has a maximum turnover number of 26 and turnover frequency of 0.063 s−1. In addition, the researchers found that this catalysis is strongly inhibited by the presence of chloride anions owing to the formation of a Cu-Cl adduct, which is not capable of generating the reactive species required for water oxidation.
- Photochemical Water Oxidation Catalysed by a Water-Soluble Copper Phthalocyanine,
Ryota Terao, Takashi Nakazono, Alexander Rene Parent, Ken Sakai,
ChemPlusChem 2016.
DOI: 10.1002/cplu.201600263 - Special Issue: Catalytic Systems for Water Splitting,
ChemPlusChem 2016, 81 (10).