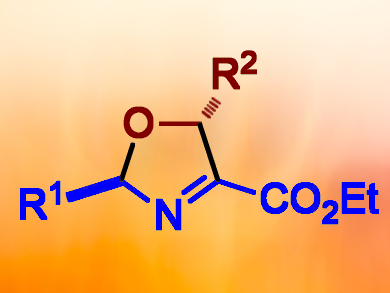
Among the motifs in heterocyclic chemistry, 3-oxazolines are of particular interest but are difficult to access. Methods for their synthesis have received little attention in comparison to that of 2-oxazolines, and a stereoselective synthesis of 2,5-disustituted 3-oxazolines has not been previously reported.
Pedro Merino and colleagues, Universidad de Zaragoza, Spain, have developed an efficient synthesis of trans-2-alkyl-5-substituted-3-oxazolines (pictured) based on an OC+CNC fragment approach. Applying a catalytic amount of nBuLi, the cycloaddition of azomethine ylide N-oxides (nitrone ylides) with aldehydes was achieved in a completely stereoselective manner. With an optimal amount of 20 mol% nBuLi, different nitrones reacted with aliphatic, aromatic, and α,β-unsaturated aldehydes.
The researchers performed mechanistic studies that provided evidence for lithium-ion catalysis. The process involves an initial nucleophilic attack on the aldehyde, followed by intramolecular addition of oxygen to the nitrone and lithium-assisted elimination of water, regenerating the catalytic lithium species. The in situ-generated water was found to be necessary for continuing the catalytic cycle. These results represent an exciting synthetic example of water and nBuLi, a water-sensitive chemical, working together.
- Azomethine Ylides from Nitrones: Using Catalytic nBuLi for the Totally Stereoselective Synthesis of trans-2-Alkyl-3-oxazolines,
Veronica Juste-Navarro, Ignacio Delso, Tomás Tejero, Pedro Merino,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201602159