The concentration of Cu2+ in blood is altered by many diseases. A noninvasive method for detecting Cu2+ would be useful to study its metabolism and understand its biological role.
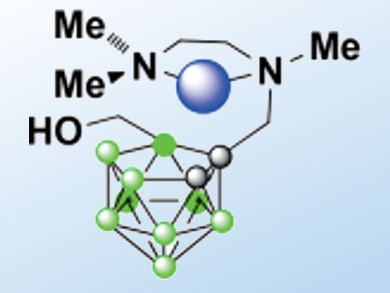
Shin Aoki, Tokyo University of Science, Japan, and colleagues developed 11B NMR probes for the detection of d-block metal ions. They synthesized a nido–o-carborane compound that contains a chelator unit, TriMEDA (pictured). As the affinity of TriMEDA for Cu2+ is higher than its affinity for Mn2+, Fe2+, or Zn2+, the Cu2+-promoted decomposition of nido–o-carborane to release B(OH)3 is accelerated considerably even at room temperature and under physiological conditions.
The released B(OH)3 can be easily and selectively detected by 11B NMR spectroscopy and magnetic resonance imaging (MRI). The cellular uptake of the nido–o-carborane compound is very low, which makes it suitable for this method. Moreover, even catalytic amounts of Cu2+ (0.1 equiv.) can be detected.
- 11B NMR/MRI Sensing of Copper(II) Ions In Vitro by the Decomposition of a Hybrid Compound of a nido–o-Carborane and a Metal Chelator,
Tomohiro Tanaka, Rikita Araki, Takaomi Saido, Ryo Abe, Shin Aoki,
Eur. J. Inorg. Chem. 2016.
DOI: 10.1002/ejic.201600346