Higher fullerenes with more than 70 carbon atoms are present in fullerene soot in very small amounts. This makes their investigation and even identification challenging tasks. However, high-temperature chlorination allows the isolation of higher fullerene chlorides in the crystalline state and their subsequent study by single crystal X-ray diffraction.
Sergey Troyanov and colleagues, Moscow State University, Russia, confirmed the presence of a relatively unstable isomer of C90, D5h-C90, in the fullerene soot produced from undoped graphite rods for the first time. They used VCl4 to chlorinate the fullerenes at 350 °C over a period of weeks.
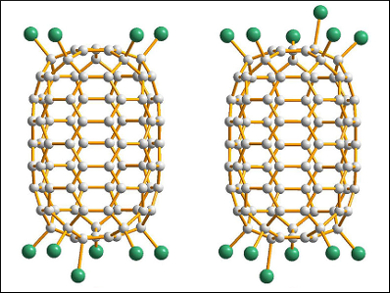
The team determined the structure of the resulting C90Cl12 (in co-crystals with C90Cl10) using synchrotron radiation. The results revealed a nanotubular cage with twelve Cl atoms, six on each end of the cage (pictured). Earlier, D5h-C90 had been isolated only from the soot produced with Sm2O3-doped graphite rods.
- Unstable Isomer of C90 Fullerene Isolated as Chloro Derivatives, C90(1)Cl10/12,
Norbert S. Chilingarov, Sergey I. Troyanov,
Chem. Asian J. 2016.
DOI: 10.1002/asia.201600713