Serine proteases are ubiquitous peptidases in which serine acts as nucleophile for a peptide bond cleavage in the enzyme’s active site. The human type II transmembrane serine protease matripase-2 plays a key role in iron homeostasis, i.e, regulating iron levels. Inhibition of matripase-2 is therefore considered an attractive target for the treatment of iron-overload diseases. However, small molecule inhibitors of matripase-2 are rare.
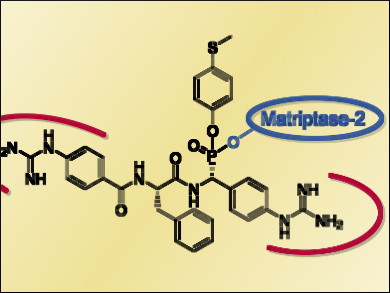
Michael Gütschow, University of Bonn, Germany, and colleagues synthesized nine different dipeptidomimetic inactivators of matripase-2. The compounds contain one phosphonate warhead (pictured blue) and two benzguanidine moieties (pictured red), which act as arginine mimetics. These groups provide affinity for matriptase-2.
Five of the compounds were identified as irreversible inhibitors of matripase-2. R-configured epimers at the phosphonate carbon atom were significantly more potent than the corresponding S-epimers. Consequently, one of the synthesized R-epimers is the most potent irreversible inhibitor of matriptase-2 known so far. The addition of coumarin as a fluorescent label into the same phosphono bisbenzguanidine inhibitor generated the first activity-based fluorescent probe for matripase-2.
The synthesized compounds might serve as a suitable starting point for the further development of irreversible matriptase-2 inactivators and as valuable tools for future investigations of the key role of matriptase-2 in iron homeostasis.
- Phosphono Bisbenzguanidines as Irreversible Dipeptidomimetic Inhibitors and Activity-Based Probes of Matriptase-2,
Daniela Häußler, Martin Mangold, Norbert Furtmann, Annett Braune, Michael Blaut, Jürgen Bajorath, Marit Stirnberg, Michael Gütschow,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201600206