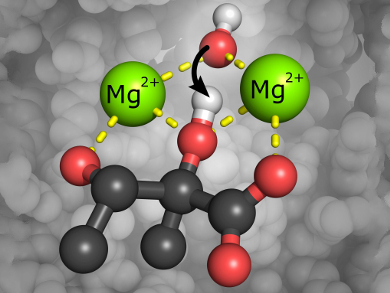
Natural ketol-acid reductoisomerases (KARIs) are enzymes that play an essential role in the branched chain amino acid (BCAA) biosynthetic pathway. Enzymes derived from this pathway have been of major interest in the development of herbicides or antimicrobials and in biofuel production. Additionally, BCAAs have received tremendous attention in recent years due to their wide distribution as sports supplements.
KARI requires two metal ions (Mg2+ or Co2+) to catalyze the conversion of BCAA intermediates by a complex two-part isomerization reaction; 1) an alkyl migration followed by 2) an NADPH-dependent reduction step. Both reactions occur within the same active site, but the mechanism of the isomerization step and the precise role of the metal ions had not been fully investigated. The Mg2+-containing enzyme is capable of catalyzing the full reductoisomerase reaction, whereas the Co2+-containing variants only accomplish the reduction step.
Luke W. Guddat, Gerhard Schenk, University of Queensland, St. Lucia, Australia, and colleagues have shed light into the KARI mechanism. They used metal-ion replacements, kinetic studies, and magnetic circular dichroism (MCD) measurements to prove that the metal ions not only play a structural role in the assembly of the enzymes active site. The ions also actively participate in the catalytic reaction by activating an essential hydroxide/water bridging unit.
The researchers also provide a possible explanation why only the Mg2+ containing enzyme variants are able to catalyze the full reaction: for metals other than magnesium, non-competent substrate binding could lead to an inhibited state of the enzyme. This could be caused by the preferred coordination chemistry of the metals.
- Metal Ions Play an Essential Catalytic Role in the Mechanism of Ketol-Acid Reductoisomerase,
Sonya Tadrowski, Marcelo M. Pedroso, Volker Sieber, James A. Larrabee, Luke W. Guddat, Gerhard Schenk,
Chem. Eur. J. 2016, 22, 7427–7436.
DOI: 10.1002/chem.201600620