Allenamides are an important synthetic building block in organic chemistry. Producing nitrogen-containing heterocycles from these precursors is of particular interest among the synthetic community. Reacting allenamides with a C1 source such as CO2 allows the synthesis of valuable amino acid derivatives.
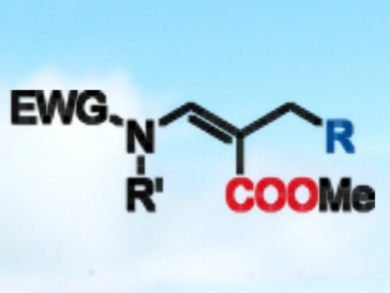
Masanori Takimoto, Zhaomin Hou, and colleagues, RIKEN, Saitama, Japan, have developed an N-heterocyclic carbene-copper complex which catalyzes the alkylative carboxylation of allenamides with CO2 and dialkylzinc reagents. Using CO2 at a pressure of 1 atm, 1.5 equiv. of dialkylzinc reagent and 5 mol% of copper catalyst, a broad scope of (Z)-α,β-dehydro-β-amino acid esters (pictured) was accessible in good yields.
The reaction has excellent regio- and stereoselectivity, with the alkyl group introduced at the less hindered allenamide γ-carbon atom, and the carboxyl group at the β-carbon. The carboxylation proceeds at room temperature and tolerates a variety of substrates, including both cyclic and acyclic compounds. The method could contribute to the implementation of greener technologies using allenamides and CO2.
- Regioselective Alkylative Carboxylation of Allenamides with Carbon Dioxide and Dialkylzinc Reagents Catalyzed by an N-Heterocyclic Carbene-Copper Complex,
Sandeep Suryabhan Gholap, Masanori Takimoto, Zhaomin Hou,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201601162