Green plants utilize water as a ubiquitous electron source for subsequent CO2 reduction in light-driven photosynthesis. The oxygen we breathe is formed as a by-product of this water oxidation reaction, which occurs at the so-called oxygen-evolving complex (OEC), an inorganic calcium manganese oxo reaction center located within a large protein assembly known as photosystem II.
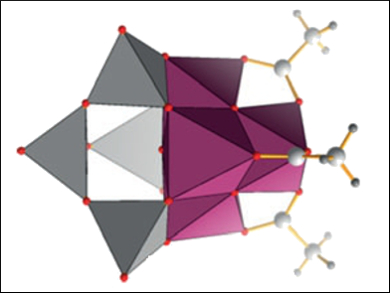
Carsten Streb and colleagues, University of Ulm, Germany, have developed a synthetic model for the OEC based on a manganese vanadium oxide polyoxometalate, [Mn4V4O17(OAc)3]3-. The compound was synthesized using a one-pot reaction of (nBu4N)4[V4O12], Mn(OAc)3 · 2 H2O, and (nBu4N)MnO4 in acetonitrile. The cluster’s structure and properties were characterized using elemental analysis, cyclovoltammetry, mass spectrometry (MS), and single-crystal X-ray diffraction.
The system allows the stabilization of the reactive manganese-oxo core in several oxidation states. Catalytic studies show that the system is capable of performing the visible-light-driven water-oxidation reaction. The study provides insight into the stabilization of bio-inspired polynuclear metal-oxo reaction sites within polyoxometalate frameworks and provides a basis for understanding the function of homogeneous and heterogeneous metal-oxide water-oxidation catalysts.
- Visible-Light-Driven Water Oxidation by a Molecular Manganese Vanadium Oxide Cluster,
Benjamin Schwarz, Johannes Forster, McKenna K. Goetz, Duygu Yücel, Claudia Berger, Timo Jacob, Carsten Streb
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201601799