Palladium-catalyzed cross-coupling reactions, such as the Suzuki, Negishi, and Stille coupling, have been versatile protocols in organic synthesis for the construction of C–R bonds (R=C, N, O, etc.).
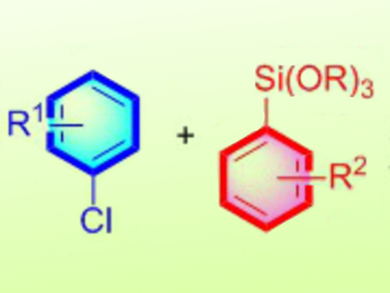
The Hiyama cross-coupling reaction is one of the most attractive methods to produce biaryl compounds, which are of significant importance to materials science. The organosilicon reagents used as coupling partners are of low cost, low toxicity, and are highly stable to a variety of reaction conditions. Aryl iodides and bromides have been predominantly used as electrophiles in the Hiyama coupling instead of the cheaper and widely available aryl chlorides. Despite recent progresses, the Hiyama reaction still suffers from limitations, such as high palladium loadings, long reaction times, and lack of the use of heteroaryl coupling partners.
Fuk Yee Kwong, Chau Ming So, Hong Kong Polytechnic University, Hong Kong, China, and collegues have developed a Hiyama cross-coupling reaction of sterically hindered aryl and heteroaryl compounds, which is challenging, with aryl and heteroaryl trialkoxysilanes (pictured). The reaction uses low loadings of palladium and the presence of water is found to remarkably enhance the reaction efficiency, leading to biaryls and heterobiaryls in good to excellent yields. According to the researchers, the water protonates an intermediate alkoxy anion, which is responsible for a competing side reaction, and thus enhances the formation of the desired product.
- A General Palladium-Catalyzed Hiyama Cross-Coupling Reaction of Aryl and Heteroaryl Chlorides,
On Ying Yuen, Chau Ming So, Ho Wing Man, Fuk Yee Kwong,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201600420