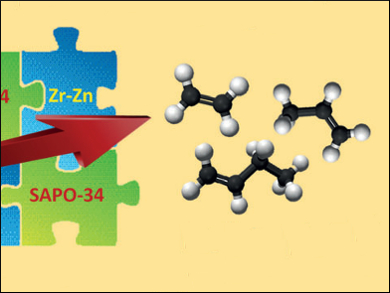
Alternative Sources for Olefin Production
The light olefins ethylene, propylene, and butylene are key building blocks for thechemical industry, and are starting materials for making plastics, synthetic fibers, and coatings. They are usually made from petroleum. In the journal Angewandte Chemie, Chinese scientists have now introduced a new process that may make it more attractive to make olefins from alternative sources of carbon, such as biomass, natural gas, or coal. The key to their success is a bifunctional catalyst that converts synthesis gas, a mixture of carbon monoxide and hydrogen, to lower olefins with astonishingly high selectivity.
Currently, the production of lower olefins primarily involves the thermal cracking of lighter fractions of petroleum. Increasing demand and decreasing oil reserves are causing scientists to turn their attention to coal, natural gas, shale gas, and biomass as sources of raw material.
The first step in such a process would involve the conversion of these materials into synthesis gas through either coal gasification or steam reforming. Using Fischer–Tropsch synthesis, the resulting synthesis gas is then catalytically converted to paraffins (saturated hydrocarbons), olefins (unsaturated hydrocarbons), and alcohols. The ratio of products follows a particular statistical distribution that limits the ability to form the desired lower olefins.
Direct Conversion of Syngas to Lower Olefins
An alternative method is the conversion of synthesis gas in two process steps. First, the CO and H2 are converted to methanol. In the second process, methanol is converted to lower olefins with relatively high selectivity by a methanol to olefins (MTO) process, which forms carbon–carbon bonds. Yet, a one-step process that combines these two processes is more desirable because it would be more energy- and cost-efficient.
Scientists working with Qinghong Zhang and Ye Wang at Xiamen University, China, have now met this challenge. Their secret is a special bifunctional catalyst with active components for both reaction steps: SAPO-34, a silicon aluminum phosphate molecular sieve, is an outstanding catalyst for the MTO reaction. Zirconium oxide and zinc oxide nanoparticles in a 2:1 ratio catalyze the methanol synthesis reaction with a preference for lower olefins.
Bifunctional Catalyst
The way in which the two components of the combined catalyst are united is of critical importance. They must be in close contact, but if they are too close then the newly formed olefins can too easily come into contact with the catalytic centers that are intended to convert the CO and hydrogen to methanol. The olefins can also bind hydrogen, which causes them to lose their double bond and thus forms more paraffins. The best results came from grinding the two catalysts together in a mortar.
At about 400 °C, the bifunctional catalyst resulted in a selectivity of 74 % for lower olefins with a CO conversion of about 11 %. This is an excellent result for the direct conversion of synthesis gas to lower olefins. The design of bifunctional catalysts is expected to result in further breakthroughs in developing one-step processes for the selective production of fuels and chemicals such as gasoline, diesel, and aromatics from synthesis gas.
- Direct and Highly Selective Conversion of Synthesis Gas to Lower Olefins: Design of a Bifunctional Catalyst Combining Methanol Synthesis and Carbon-Carbon Coupling,
Kang Cheng, Bang Gu, Xiaoliang Liu, Jincan Kang, Qinghong Zhang, Ye Wang,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201601208