The introduction of fluorine groups into organic compounds can significantly change their chemical and physical properties. Amongst numerous fluorination strategies, trifluoromethylthiolation is of significant interest, especially for pharmaceuticals. The trifluoromethylthio group is highly lipophilic, which improves membrane permeation when incorporated into bioactive molecules. A majority of approaches to the synthesis of trifluoromethylthio compounds make use of transition-metal catalysis and is focused on aryl and heteroaryl compounds.
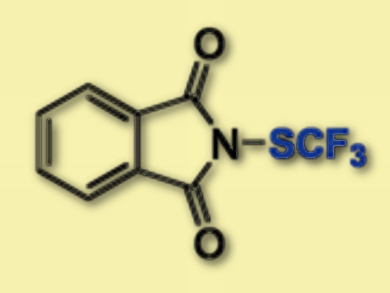
Frank Glorius, Mathew N. Hopkinson, and colleagues, University of Münster, Germany, have developed an approach for the trifluorormethylthiolation of styrenes using visible-light-promoted dual photoredox/halide catalysis to give vinyl-SCF3 compounds in high yields. The reaction is based on halide activation of N-(trifluoromethylthio)phthalimide (pictured).
The method is also capable of delivering alkyl-SCF3 compounds containing an all-carbon quaternary stereocenter that are usually difficult to synthesize. The team conducted mechanistic investigations and halide salts were found to play a crucial role in activating the trifluoromethylthiolating reagent towards photoredox catalysis and aid the formation of the SCF3 radical.
- Visible-Light-Promoted Trifluoromethylthiolation of Styrenes by Dual Photoredox/Halide Catalysis,
Roman Honeker, R. Aleyda Garza-Sanchez, Matthew N. Hopkinson, Frank Glorius,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201600190