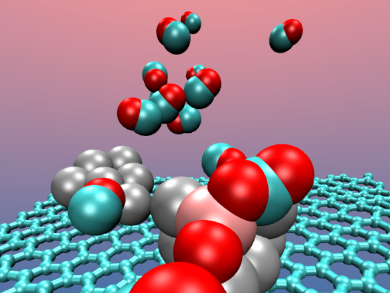
Katsuyuki Nobusada and colleagues, University of Kyoto, Japan, have investigated the oxidation mechanisms of CO to CO2 on graphene-supported Pt and Pt-Al alloy clusters by reactive dynamical simulations. A Langmuir–Hinshelwood (LH) mechanism in which O2 is adsorbed on the cluster prior to the CO oxidation was found. The adsorbed O2 dissociates and promotes the CO oxidation.
The researchers used a Car-Parrinello molecular dynamics approach based on Density Functional Theory (DFT) for their investigation. Simulations on alloy clusters in which metals such as Al, Co, Cr, Cu, Fe, and Ni replaced a Pt atom showed best results for the aluminum doped cluster.
In the Pt-Al nanoalloy, the reaction mechanism is still a LH pathway with an activation barrier sufficiently low to be overcome at room temperature, thus preserving the catalyst efficiency. The aluminum plays an active role and acts as the attractive site for the oxygen adsorption in the first stage of the reaction process.
This insight provides a generalizable strategy for the design of efficient, yet sustainable, Pt-based catalysts at reduced cost.
- Reducing the Cost and Preserving the Reactivity in Noble-Metal-Based Catalysts: Oxidation of CO by Pt and Al–Pt Alloy Clusters Supported on Graphene,
Kenichi Koizumi, Katsuyuki Nobusada, Mauro Boero,
Chem. Eur. J. 2016.
DOI: 10.1002/chem.201504379