Targeting Enzymes Involved in Type 2 Diabetes
A freshwater fungus found in Japan has yielded a rather unusual natural product. The compound, an ether, has been shown to have potent activity against the enzyme protein tyrosine phosphatase 1B (PTP1B) and thus holds promise as a novel treatment for type 2 diabetes, say its discoverers.
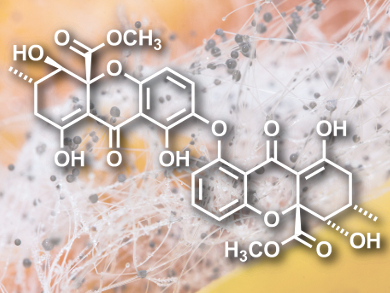
Hiroyuki Yamazaki, Kazuyo Ukai, and Michio Namikoshi of Tohoku Pharmaceutical University, Sendai, Japan, found the bis-tetrahydroxanthone (pictured), which they dubbed asperdichrome, in a culture broth of the freshwater fungus Aspergillus sp. TPU1343 from Iriomote Island, Okinawa, Japan.
Previously, the team searched among marine organisms such as sponges, tunicates, and microorganisms, looking for novel natural products that might be involved in enzymes targeted in type 2 diabetes. They found polybromodiphenyl ethers, dehydroeuryspongin A, hyattellactones, trichoketides, verruculides, and 26-O-ethyl-strongylophorine-14 in marine sponges and marine fungi. However, it was TPU1343 that has now yielded a compound, asperdichrome, with potent activity, an IC50 of just 6 μM against 1B (PTP1B).
PTP1B is an important negative regulator of insulin signaling pathways and, as Yamazaki and colleagues explain, has been implicated in recent studies of the body’s hormonal food satiety system, the signaling cascade. More than 300 inhibitors of this enzyme have been identified so far from natural sources and investigated with regard to addressing the growing problem of obesity, type 2 diabetes, and metabolic disorders. Unfortunately, activity and selectivity have not been sufficiently high to lead those natural products down the clinical path to approved medication.
Pinning Down the Molecular Structure
As with many drug discovery stories, molecular diversity, unusual structural skeletons, and intriguing activity in the organism of origin often point the way towards useful drug leads. As such, the team was keen to study asperdichrome, a novel compound from a rarely encountered fungus. Related tetrahydroxanthones were originally isolated from ergot (Claviceps purpurea), the toxic rye fungus, and from Penicillium oxacum, which produces the compound secalonic acid B. The secalonic acids are dimers of tetrahydroxanthone, in which the bridge is a biphenol rather than an ether link.
The team used the usual raft of analytical techniques – nuclear magnetic resonance (NMR), UV-Vis, infrared (IR), and circular dichroism spectroscopy – to determine the structure of the novel ether and showed it to be similar to two secalonic acids which were present in the broth in that it is a dimer of an tetrahydroxanthone, albeit bridged with an ether link. Additional proton-proton COSY (correlation spectroscopy) and HMBC (heteronuclear multiple-bond correlation spectroscopy) NMR spectra pinned down the details and revealed the presence of 1,2,3-trisubstituted and 1,2,3,4-tetrasubstituted aromatic rings and two cyclohexene moieties in asperdichrome.
Natural Comparisons
The team compared the asperdichrome and the two secalonic acids from the broth in biological tests. They found that asperdichrome was the most active, but one of the secalonic acids was close to that activity with an IC50 of 9.6 μM as opposed to the 6 μM observed for asperdichrome. However, the other secalonic acid present was only 40 % active even at 15.7 μM. “Tetrahydroxanthones are a new type of PTP1B inhibitor, and, thus, further evaluations as new lead compounds for the development of drugs for type 2 diabetes and obesity are expected,” the team concludes.
Other international teams have also sought PTP1B inhibitors from various natural sources and some have been successful in their endeavors. “Finding activity, even potent activity, was not a problem, but getting the specificity that would be required to be a realistic drug lead was,” Alan Harvey, Strathclyde Institute of Pharmacy and Biomedical Sciences, Glasgow, UK, told ChemViews Magazine.
Issues that remain to be addressed in this area are not only activity against the target enzyme but specificity, so that the inhibitor does not interfere with related enzymes. Moreover, it remains to be seen whether or not PTP1B is a tenable target for a diabetes treatment in the first place and whether an inhibitor will have the desired effect of taking control of blood sugar in clinical practice.
- Asperdichrome, an unusual dimer of tetrahydroxanthone through an ether bond, with protein tyrosine phosphatase 1B inhibitory activity, from the Okinawan freshwater Aspergillus sp. TPU1343,
Hiroyuki Yamazaki, Kazuyo Ukai, Michio Namikoshi,
Tetrahedron Lett. 2016, 57, 732–735.
DOI: 10.1016/j.tetlet.2015.12.111