Nitrogen containing molecules are of great interest in the biological, pharmaceutical and materials sciences, so the controlled and catalyzed introduction of a nitrogen atom into a synthetic target has undergone much research.
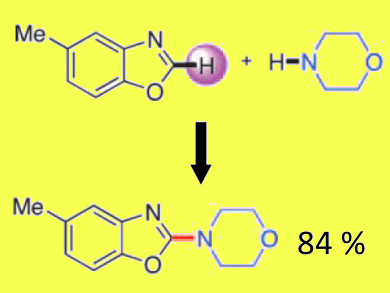
Sukbok Chang and co-workers, Korea Advanced Institute of Science and Technology, Republic of Korea, have examined the amination of azoles in the presence of a peroxide-based oxidant, a Brønsted acid and cobalt or manganese acetate. By using these conditions, the direct amination of benzoxazoles could be carried out at room temperature, with low catalyst loadings (2 mol%), to afford a range of 2-aminated products.
From kinetic isotope effects and isolation of amidine compounds, the authors propose the in situ formation of alkoxy and alkylperoxy radicals, each of which abstracts a hydrogen atom from the 2-aminobenzoxazolidine intermediate to produce the 2-aminobenzoxazole product.
- Cobalt- and Manganese-Catalyzed Direct Amination of Azoles under Mild Reaction Conditions and the Mechanistic Details
J. Y. Kim, S. H. Cho, J. Joseph, S. Chang,
Angew. Chem. Int. Ed. 2010, 49.
DOI: 10.1002/anie.201005922 - J. Y. Kim, S. H. Cho, J. Joseph, S. Chang,
Angew. Chem. 2010, 122.
DOI: 10.1002/ange.201005922